Sulphate in River Spree and Lake Müggelsee
Short profile
Duration

Lake Müggelsee. The drinking water reservoir of Berlin, is loaded with sulfate from the Lusatia. (Photo: Thomas Rossoll)
Data: Current sulfate concentration and long-term development
background
For decades, groundwater levels in Lusatia were lowered for mining. The uplifted and then led into the Spree groundwater has very high concentrations of dissolved divalent iron, it was then as today in mine water treatment plants by appropriate precipitant iron-free and additionally neutralized.
In the waste dumps and in the entire groundwater lowering funnel, the formerly water-saturated and oxygen-free soil areas were ventilated - sometimes to a depth of more than 100 meters. The mineral pyrite present in these layers (an iron sulfide) is thereby oxidized, which is described in the following chemical equation:
![]()
Since most of the old moraine areas of Lusatia are very low in lime and thus lacks the carbonate buffer otherwise present in many regions of the late pleistocene, the sulfuric acid formed can not be bound. As a result, the increase in groundwater in the vicinity of abandoned opencast mines - intensified since 1990 - led to massive acidification of the lakes. The problem of acidification was also observed in various tributaries of the Spree. In addition, the sulfate and iron concentrations increased: In the new, partly large mountain reservoirs that dewater to the Spree, the sulfate concentrations are up to 3000 mg / L, that of iron at up to 500 mg / L. As soon as the pH rises again in rivers or lakes through natural processes or technical measures, readily visible iron (III) hydroxides are precipitated ("browning"). The invisible sulphate, however, remains in the water and is transported downstream, which leads to a decoupling of the sulfur and iron cycle. Details have already been described in
Dossier: "Sulfatbelastung der Spree - Ursachen, Wirkungen und aktuelle Erkenntnisse“ (in german only)
How this affects the long-term development of sulphate concentrations at selected measuring points of the River Spree and in the Lake Müggelsee will be shown in the following investigations.

Lake Müggelsee. The drinking water reservoir of Berlin, is loaded with sulfate from the Lusatia. (Photo: Thomas Rossoll)
Opencast mine hole in the Lausitz is filling (Photo: Jörg Gelbrecht)

Brown run-off from mining lake (Photo: Dominik Zak)
The 1,900-hectare lake "Cottbus Baltic" is created from a former brown coal mine. (Photo: Jörg Gelbrecht)
Another source for the rising sulfate in River Spree. Fens were drained for agricultural use (Demnitzer Mühlenfließ east of Fürstenwalde, 2002).(Photo: J. Gelbrecht)
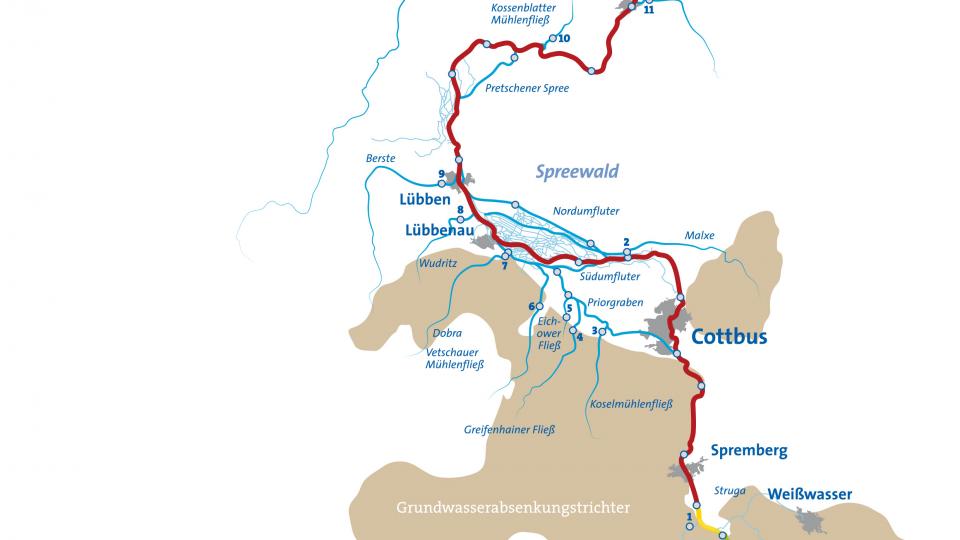
Concentration of sulphate in the course of the Spree. (Source: IGB Dossier: Sulfatbelastung der Spree)

In the long term, old structures and infrastructures can be damaged by the penetration of sulphate-containing water. (Photo: Thomas Rossoll)






